![]()
you already know: - general explanation for: micromixing and segregation
The basis for the explanation is the formula of the
Area-method and the idea for making the formula
comprehensible. Let us assume that our reaction takes place in a CSTR and let
us take a molecular dispersed fluid (microfluid) for the first. The
concentration of the educt will drop down to the final value (due to residence
time -> conversion) at once. When we take a
remarkable conversion the final concentration will be very low and the reaction
rate for the second order reaction will be even lower. When we take a
segregated fluid on the other hand, we have an 'infinitesimal crowd of
micro-batch-STRs', each with an individual residence time in the 'big pot'. The
concentration decay of the educt within each micro-STR follows the time
'caused' by batch reaction kinetics. It is clear that we will encounter
a higher mean concentration of educt in this case:
the reaction rate (r proportional to Cn) - and as a consequence the
space time yield- will be of a higher mean value.
*
Let us try to visualize that with two
sketches:
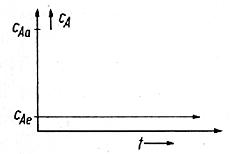
let us first look at the decomposition reaction of a red dye
that is carried out in a molecular dispersed fluid in a CSTR: the reaction rate
is proportional to the very low 'rest concentration' of the dye in the whole
vessel, the space time yield is low, high conversions can only be reached with
high space times. This could look a bit like the following:
remeber:
On the other hand let us imagine that the fluid is segregated, this could look a bit like in the following image, when we enlarge the micro-STR's with a virtual zoom:
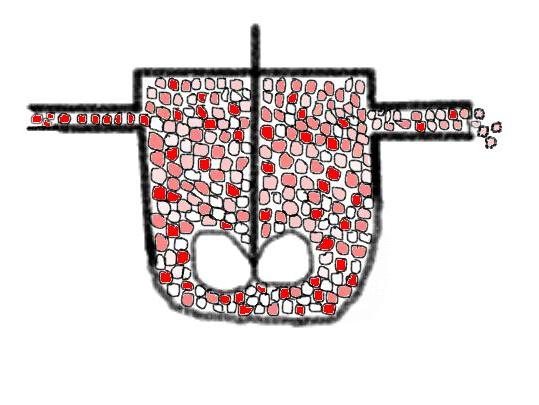
you see that the mean dye colour is a bit more intense
here than that from the image above. This means that we have a bit a higher
mean driving force 'educt concentration' in this case. Perhaps you have to bat
a bit your eyes to get an average colour impression (part of the problem that
the blending effect is hindered are the black boundary lines of the
micro-STR's, when you imagine that they are removed you would get a
'pointilistic' image (melting the colours) as a better
visualization).
Better try it with the following
copies:
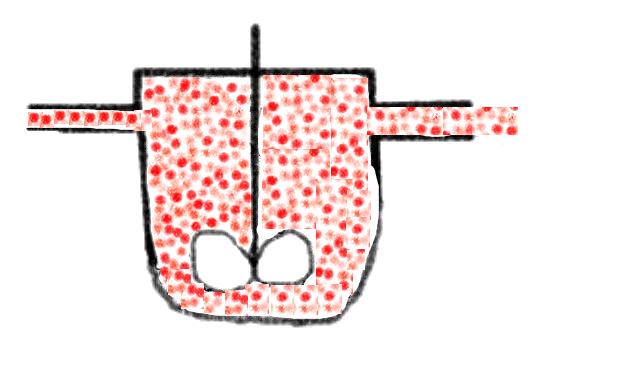

Perhaps it is also useful to zoom virtually into the 'spot' where the inlet of the educt stream takes place:
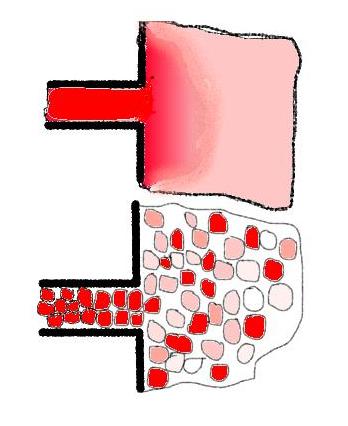
the upper sketch shows the molecular dispersed fluid,
here is the inlet colour stream diluted nearly at once and the stationary
colour is reached
the lower sketch shows schematic (zoomed) for the
segregated fluid that the total back-mixing effect of the CSTR produces indeed
a complete mixing of the fluid, but as the micro-STR's don't 'interchange',
their 'interior' rests unaffected by the back-mixing.
take your browser back for previous text or: