look at two sketches
 for
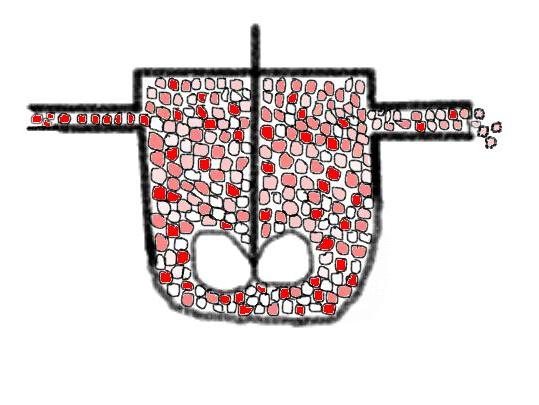
the visualization of molecular dispersed and segregated fluids In CSTR's
!!
for
the visualization of molecular dispersed and segregated fluids In CSTR's
!!*micro-mixing - as we define it in this course - should now first be explained
Contrary to macro-mixing
(contacting pattern) that describes the macroscopic mixing-effects in the
reaction vessel as we could see it with our 'bare eyes', micro-mixing is a feature
of the fluid: the fluid can either be mixed in a molecular dispersed
manner (microfluid, e.g. salt solution, sugar solution etc.) or on the
other hand it can be totally segregated ( macrofluid in english
literature).
Segregation can be represented by different models:
recommended!!
look at two
sketches
 for
the visualization of molecular dispersed and segregated fluids In CSTR's
!!
for
the visualization of molecular dispersed and segregated fluids In CSTR's
!!
As it can not be expected that we find very often totally segregated systems in reality, we can postulate a degree of segregation, that means that 'coalescence' (i.e. interchange) takes place to a certain percentage
For the difference between our definitions and those that are found in international literature see: (overview)
back to top
take your browser back for previous text or: