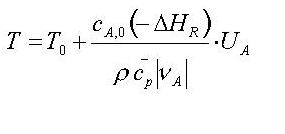
If you are solving the heat balances of all three basic
reactor types for the adiabatic case, you get 'amazingly' the same
solution: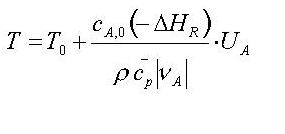
Do you know that the conversion U is coming from the reaction term and are you able to derive this formula ? Not ? (word.doc derived htm!!)
If you write: T = T0 + Tad * U
A
you see the definition of Tad:
Tad
is the temperature that can be reached in an adiabatic reaction for full
conversion, it is the maximum of heat you can expect in this case, it depends
mainly on the reaction enthalpy. For lower conversions you get a 'partial'
adiabatic temperature rise following the formula. The adiabatic temperature
rise is a linear function of the conversion (don't change that with the
reaction time, as U is not a linear function of reaction time!!)
see link!!
take your browser back for previous text or: