 to main test page
to main test page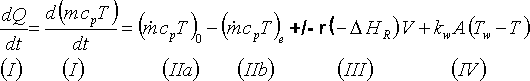
Term (I) = instationary term, i.e. observable change in heat (or temperature) that 'goes into' the reaction mass.
Term (II) = convective term = difference between 'incoming' and 'outgoing' heat
Term (III) = reaction term = heat produced or consumed by the chemical reaction
Term (IV) = heat exchange term = heat exchange by exchanging processes (devices)
take your browser back for previous text or: